The Essential Role of Calcium in Biological Fitness
Calcium plays a fundamental role in maintaining biological fitness, primarily as a divalent cation (Ca²⁺) involved in an extensive range of cellular and physiological activities. It is actively transported out of the cytoplasm into either the extracellular space or specialized internal reservoirs. Upon receiving external stimuli, eukaryotic cells release calcium from these stores into the cytoplasm or organelles, initiating a wide array of essential processes. Primary signaling molecule: Calcium acts as a vital first messenger in cellular signaling pathways, converting external cues into coordinated intracellular responses. Regulator of physiological responses: It is indispensable in muscle contractions, secretion of digestive enzymes (such as from the pancreas), and hormone release from various glands. Structural component: Calcium is the major extracellular cation involved in stabilizing protein structures and is a key element in biomineralization, contributing to the formation of bones, teeth, and shells. Calcium is a chemical element with atomic number 20, classified in group 2 of the periodic table, and lies in the third period, just before the transition metals. It is positioned below magnesium and above strontium and barium. Due to its low ionization potential, calcium readily forms the divalent cation Ca²⁺, which has a relatively large ionic radius (1.0 Å). While Ca²⁺ is not a particularly strong Lewis acid and thus not a highly effective acid catalyst, it can still form strong bonds with specific anionic centers. Periodic Characteristics: Calcium’s ion chemistry aligns more closely with that of strontium (Sr²⁺) than with the smaller and more acidic magnesium ion (Mg²⁺), despite being in the same group. Calcium is abundantly present in both the universe and Earth's crust. Its compounds, like those of magnesium, are often highly soluble in water. In seawater: ~10² M (compared to magnesium at 5 × 10² M), In freshwater: Can reach concentrations as high as 10³ M. These environmental calcium levels, especially in the oceans, have influenced the evolutionary adaptation of life forms over geological time. Calcium has three main isotopes (⁴⁰Ca, ⁴²Ca, and ⁴⁴Ca) that are useful for tracing ionic activities. In biological research, calcium ions can be tracked using fluorescent organic dyes that bind selectively to Ca²⁺, allowing real-time monitoring of cellular calcium dynamics. Together, these properties make calcium a critical player in both geochemistry and cellular biology. In aqueous environments, calcium ions (Ca²⁺) are surrounded by a dynamic hydration shell. Unlike smaller cations, calcium does not have a fixed number of tightly bound water molecules. Instead, the number of water molecules coordinated around Ca²⁺ typically fluctuates between six and nine. This flexible hydration behavior is a defining feature of cations with ionic radii greater than 0.8 Å. Calcium ion radius: ~1.0 Å Hydration number: Variable, generally between 6 and 9 water molecules This flexible shell contrasts with that of magnesium, whose smaller size results in a fixed and rigid coordination of 6 water molecules. Calcium (Ca²⁺): Rapid exchange rate, near the diffusion limit at approximately 10⁹ s⁻¹, Magnesium (Mg²⁺): Much slower exchange rate, about 10⁶ s⁻¹. These distinctions in hydration behavior and exchange kinetics between calcium and magnesium ions are important in biological systems, influencing how each cation participates in signaling, transport, and enzymatic interactions. The interaction of calcium ions with various anions plays a critical role in determining the solubility of salts in both environmental and biological systems. Due to its larger ionic radius, calcium shows unique solubility characteristics when compared with magnesium, especially in the context of biological precipitation. Calcium, being a larger ion, binds less strongly to small anions such as fluoride or hydroxide than magnesium does. This makes magnesium salts of small anions typically less soluble than their calcium counterparts. On the other hand, larger anions like carbonate, sulfate, and phosphate better accommodate the spatial needs of the calcium ion. This results in calcium salts of these anions being less soluble than their magnesium analogs. Calcium carbonate is a principal component of shells in marine organisms. Calcium phosphate forms the structural matrix of bones and teeth in vertebrates. Calcium oxalate commonly precipitates in plant cells, often appearing as crystals. Abnormal calcium precipitation can lead to the formation of kidney and bladder stones in animals. In geological formations such as dolomite, calcium and magnesium frequently appear together, reflecting their widespread natural distribution and chemical similarity. Calcium ions generally do not form strong complexes with simple anions such as carbonate, sulfate, hydroxide, or phosphate in aqueous environments at neutral pH. Consequently, these interactions are not particularly impactful in biological systems. However, when calcium binds to ligands that feature multiple donor atoms—especially those large enough to accommodate its ionic size—the resulting complexes can be significantly more stable. Calcium forms strong bonds with multidentate ligands due to its larger ionic radius, allowing better spatial fit and coordination. In contrast, magnesium ions (Mg²⁺), being smaller and more rigid, bind weakly with the same ligands. As a result, chelating agents with multiple binding sites tend to prefer calcium over magnesium by a factor of at least 1000. This selectivity is critical in biological systems where specific calcium-binding proteins, both intracellular and extracellular, are involved in regulation. An example is ethylene tetra-acetate (EDTA), a calcium buffer that exhibits strong affinity for calcium ions while showing minimal interaction with magnesium at physiological pH. This binding behavior underpins many regulatory and buffering roles that calcium plays in living organisms, distinguishing it functionally from other divalent cations like magnesium. The structural diversity of calcium salts and their complexes is considerable, influenced by both the nature of the anion and the presence of coordinating ligands. Studies on crystalline forms of calcium salts have revealed distinct coordination patterns: Calcium carbonates exhibit polymorphism, with calcite displaying a six-coordinate lattice and aragonite showing a nine-coordinate structure. Both are anhydrous and are commonly found in natural structures like seashells. In aqueous solution, particularly when bound to bulky chelating organic ligands, calcium often adopts a seven-coordinate geometry. However, the Ca–ligand atom distances are not uniform and can range from 2.2 Å to 3.0 Å, depending on the ligand's nature and flexibility. When calcium ions are coordinated with saccharides or proteins, this general seven-coordinate pattern persists, though the structure is dynamic and subject to fluctuation. One or two water molecules are often retained in the coordination sphere. The donor atoms in these complexes are mainly oxygen atoms, derived from various functional groups such as: Oxyanions Carboxylates Alcohols Carbonyl groups Ethers This versatile coordination behavior allows calcium to participate in a wide array of biological structures and interactions, underscoring its importance in both structural and signaling roles in living organisms. The rate at which calcium ions interact with ligands plays a pivotal role in their biological signaling function. In aqueous environments, Ca²⁺ ions lose and gain water molecules at an exceptionally fast rate—approximately 10⁹ s⁻¹. This rapid hydration exchange rate aligns with the association rate constant (kon) for ligand binding. The ligand binding constant (K) is defined by the ratio K=kon /koff If K = 10⁶ M⁻¹ (a typical value in biological systems), then the dissociation rate (koff) is 10³ s⁻¹. These kinetics are mirrored in protein-calcium interactions. A classic case is calmodulin, a calcium-binding messenger protein that responds at a rate of 10³ s⁻¹, which supports high-speed cellular processes such as those found in fast-twitch muscle fibers. Such rapid on/off rates, combined with moderate binding affinities, make calcium an ideal signaling ion, allowing for precise, transient responses in biological systems. The exceptional exchange dynamics of calcium with ligands enable it to function as a highly responsive and versatile messenger, essential for a broad range of physiological events. Calcium forms several important insoluble salts and protein-ligand complexes that are essential to biological structure and function: Fluoride: Found in krill and contributes to mineralization. Carbonates: Occur in shells with at least three different allotropic forms, such as calcite and aragonite. Phosphates: Form the primary component of bones, specifically as hydroxyapatite, a non-stoichiometric mineral that easily incorporates other ions like magnesium and fluoride. Oxalates: Commonly found in plants and often precipitate as part of calcium detoxification or storage. Its composition differs by species. Even within the same organism (e.g., humans), variations exist between teeth and bones. Calcite is found in animal ears, serving a sensory role in equilibrium and balance. In cells, calcium ions are coordinated with seven oxygen-donor groups from proteins and polysaccharides. These donor atoms include side-chain and main-chain groups such as hydroxyl (-OH), carbonyl (CO), carboxylate (CO₂⁻), ether, and sulfate (SO₄²⁻) groups. Calcium shows minimal interaction with nucleic acids (DNA and RNA), a notable contrast to magnesium, which plays a vital role in their structural stability. This selective binding is driven by differences in cellular concentrations and the coordination preferences of each metal ion. In essence, calcium's structural roles span from mineralized tissue formation to intricate protein-ligand interactions, while its selective chemistry defines its specific niche in biological systems. The unique physiological behavior of calcium in living organisms stems from its precise intracellular regulation, selective binding characteristics, and ideal biochemical kinetics. Early life had to evolve mechanisms to control intracellular calcium due to its tendency to precipitate organic anions like DNA. This led to the following principles: In prokaryotes and all living cells, Ca²⁺ is maintained below 10⁻⁶ M through the action of calcium-extruding pumps. This prevents calcium from forming insoluble complexes with negatively charged biomolecules like nucleic acids. Mg²⁺ does not precipitate organic anions, so it is tolerated at higher cytoplasmic levels (~10⁻³ M). For calcium to bind selectively inside cells, its binding constant (K) must be ≥ 10⁶ M⁻¹, whereas magnesium must bind weakly (K < 10³ M⁻¹). This ensures selective recognition by messenger proteins like calmodulin, troponin, and S-100, which respond only to calcium. Calcium does not bind well to ATP or DNA (K ≈ 10⁴), unlike Mg²⁺, which binds them effectively at similar constants. Outside cells and in vesicles, both Ca²⁺ and Mg²⁺ are found at >10⁻³ M, but calcium can still bind selectively due to stronger affinities (K ~10³ M⁻¹ vs. Mg²⁺ < 10² M⁻¹). In resting cells, cytoplasmic Ca²⁺ is ~10⁻⁷ M, while extracellular or vesicular Ca²⁺ is ~10⁻³ M. When calcium channels open, Ca²⁺ levels transiently rise to ~10⁻⁶ M, activating intracellular signaling proteins. Calcium pumps restore resting levels, allowing for precise “pulsed” signaling The association (kon) and dissociation (koff) rates of calcium with target proteins support rapid, reversible binding, often ≥ 10³ s⁻¹. This enables timely cellular responses, particularly in processes like muscle contraction, vesicle release, and immune activation. Calcium's low resting concentration, tight binding selectivity, controlled compartmentalization, and fast exchange kinetics make it uniquely suited as a biological messenger ion. No other ion exhibits this combination of properties, which explains its central role in cellular signaling across all forms of life. The evolutionary fitness of calcium ions lies in their exceptional chemical versatility and biological accessibility. Their ability to rapidly associate and dissociate from organic ligands—thanks to a flexible coordination sphere—makes calcium an ideal messenger and structural ion across all domains of life. In the earliest life forms, calcium supported structural integrity (e.g., cell walls) and played catalytic roles in digestive enzymes. These foundational roles persisted across evolutionary lines. As eukaryotes emerged from prokaryotes, the need for complex intracellular coordination and rapid environmental responsiveness became critical. Calcium ions filled this niche due to: High natural abundance. Tight regulation mechanisms. Fast, reversible binding to key proteins. Cytoplasmic receptor proteins, such as troponin, calmodulin, S-100, and calcineurin, evolved to alter conformation upon calcium binding, thereby: Controlling muscle contraction. Initiating fertilization. Triggering gene expression. Activating immune signaling. Calcium concentration homeostasis advanced significantly in humans, allowing: Tight control in cytoplasm and external fluids. Support for immune responses, blood clotting, and digestive enzyme release. Calcium pulses initiate ATP production in mitochondria and chloroplasts. Hormone secretion and neurotransmitter release via vesicle-mediated signaling. Broad regulatory effects on homeostasis, metabolism, and neural activity. Bone acts as a long-term calcium buffer, stabilizing systemic calcium levels and providing a reserve during metabolic demand. Calcium ions have become central to cellular signaling and systemic regulation, serving not just as structural components but also as evolution’s most effective messenger system. From ancient enzymes to modern neural synapses, calcium's role has been refined and diversified, culminating in its indispensable contributions to both simple and complex life. Calcium stands out as a uniquely versatile and indispensable element in biology, fulfilling structural, regulatory, and signaling roles across all forms of life. From its early functions in cell walls and enzymatic catalysis to its pivotal role in coordinating complex cellular responses in eukaryotes, calcium has proven to be a cornerstone of biological fitness. Its ability to bind selectively, respond rapidly, and be tightly regulated makes it the ideal messenger for transmitting signals within and between cells. Whether initiating muscle contraction, activating immune responses, or regulating metabolic processes, calcium ions serve as a master communicator in cellular physiology. Furthermore, the long-term buffering capacity provided by bone and the precise modulation of calcium levels in cytoplasm and extracellular fluids underscore its systemic importance. In essence, the evolutionary success of multicellular organisms, including humans, has been profoundly shaped by the dynamic and multifaceted roles of calcium in maintaining homeostasis and enabling biological responsiveness. Calcium plays a vital role in both structural and functional aspects of the body. Structurally, it is a key component of bones and teeth in the form of calcium phosphate. Functionally, it regulates muscle contractions, blood clotting, nerve signal transmission, enzyme activity, and hormone secretion. Its ability to act as a signaling messenger inside cells makes it essential for maintaining biological fitness and homeostasis. Calcium ions (Ca²⁺) function as a fast-acting, reversible intracellular messenger. When stimulated, calcium channels open, allowing Ca²⁺ to enter the cytoplasm from extracellular fluid or internal stores. This temporary rise in calcium concentration triggers specific proteins—such as calmodulin and troponin—which then activate muscle contractions, hormone release, or metabolic changes. Once the signal is delivered, calcium is rapidly pumped back to maintain resting levels, enabling repeated pulses of activity. In bodybuilding, calcium is essential for muscle contraction and recovery. Calcium enables the interaction between actin and myosin filaments during muscle contraction. Without adequate calcium, muscle function weakens. Furthermore, calcium helps regulate hormonal signals involved in muscle repair and growth. Athletes also require calcium to support strong bones, preventing injuries during intense training. Calcium regulation is critical because even small fluctuations in cellular calcium levels can have significant effects. High intracellular calcium can cause unwanted precipitation of organic molecules, while too little impairs essential cellular functions. Therefore, cells maintain low cytoplasmic calcium levels (about 10⁻⁷ M) and use specialized proteins and organelles to tightly control calcium release, binding, and removal. Calcium serves a dual purpose: Structural: Forms the mineral matrix of bones (hydroxyapatite) and hard tissues like shells and teeth. Signaling: Acts as a dynamic messenger involved in numerous processes such as muscle contraction, enzyme activation, neurotransmitter release, and immune response. This dual role highlights calcium’s evolutionary adaptation for both stability and rapid cellular communication.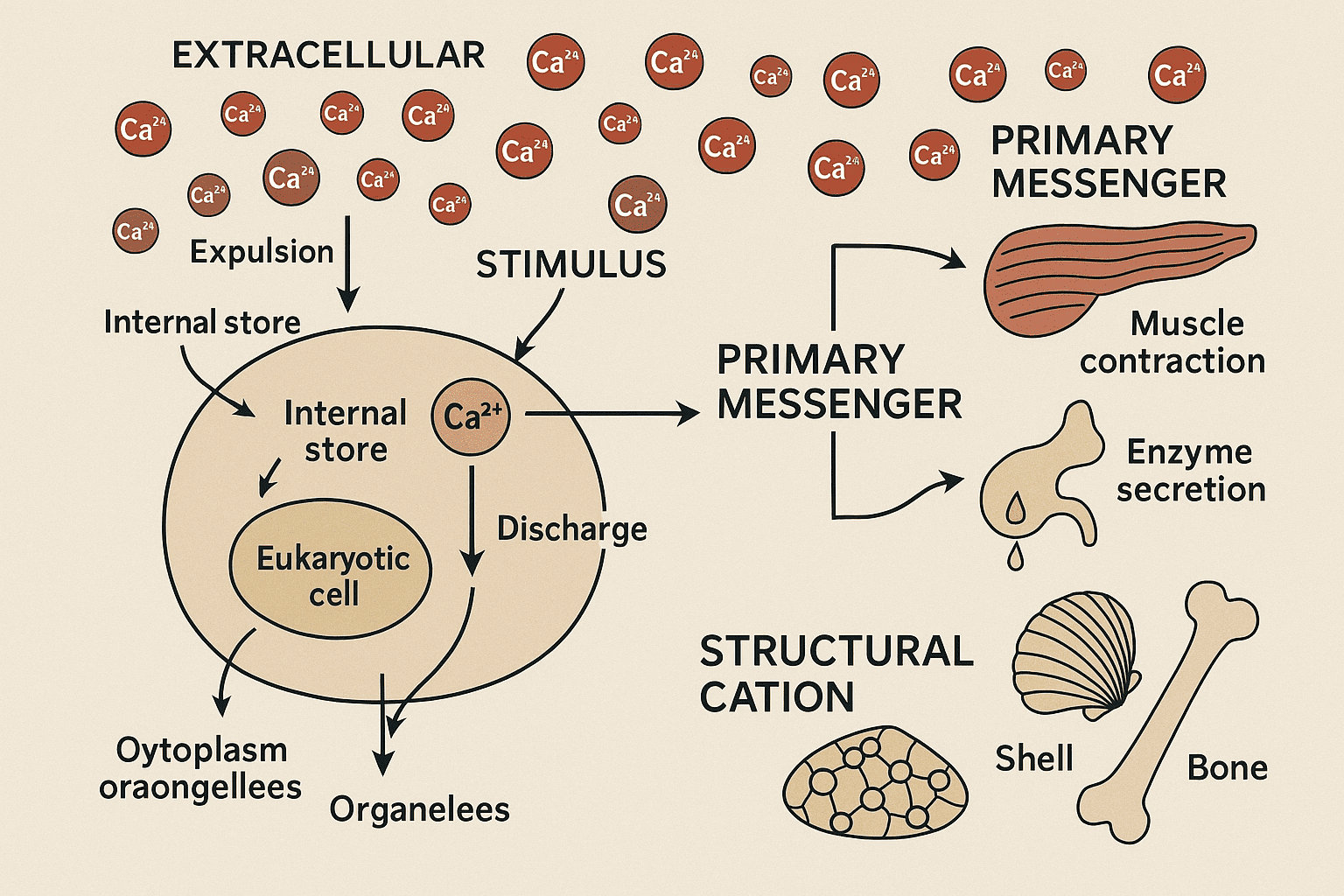
1. Introduction
2. The Element Behind the Ion Ca²⁺ and Its Significance
2.1 Abundance and Solubility:
Typical concentrations:
2.2 Isotopes and Detection:
3. Hydration Behavior and Dynamics of Aqueous Calcium Ions
3.1 Hydration Shell Characteristics:
3.2 Water Exchange Dynamics:
4. Calcium Salt Formation and Solubility in Biological Systems
4.1 Ionic Size and Anion Interaction:
4.2 Affinity for Larger Anions:
4.3 Biological Implications:
4.4 Natural Co-occurrence:
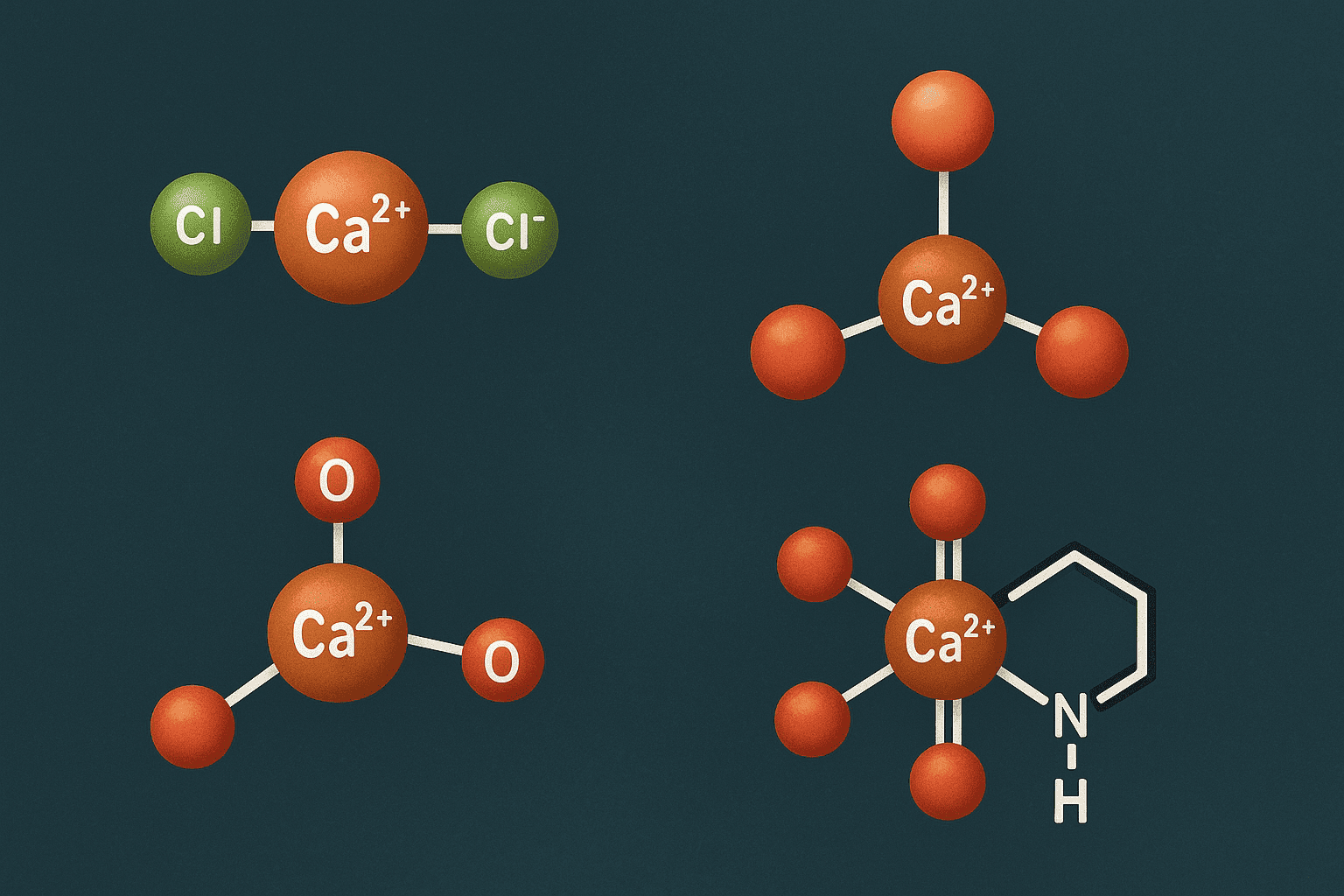
5. Calcium Ion Binding: Complex Formation and Selectivity
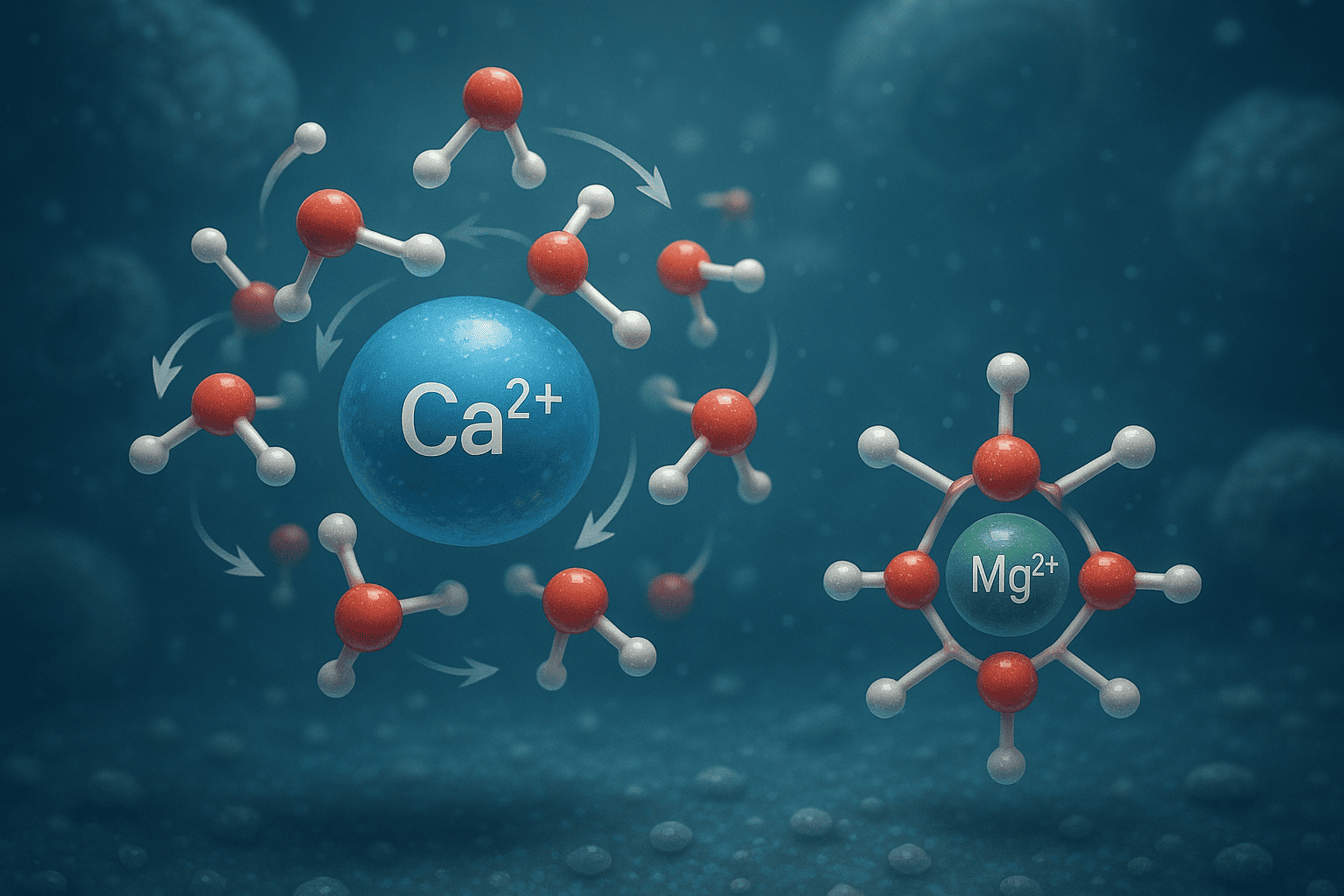
6. Structural Variability in Calcium Salts and Organic Complexes
7. Kinetics of Calcium-Ligand Exchange: The Basis for Its Signaling Efficiency
For example:8. Biological Calcium Salts and Protein Complexes
8.1 Key Insoluble Salts in Biology:
8.2 Hydroxyapatite (bone mineral) is complex and varies:
8.3 Protein and Polysaccharide Complexes:
8.4 Selective Binding Behavior:
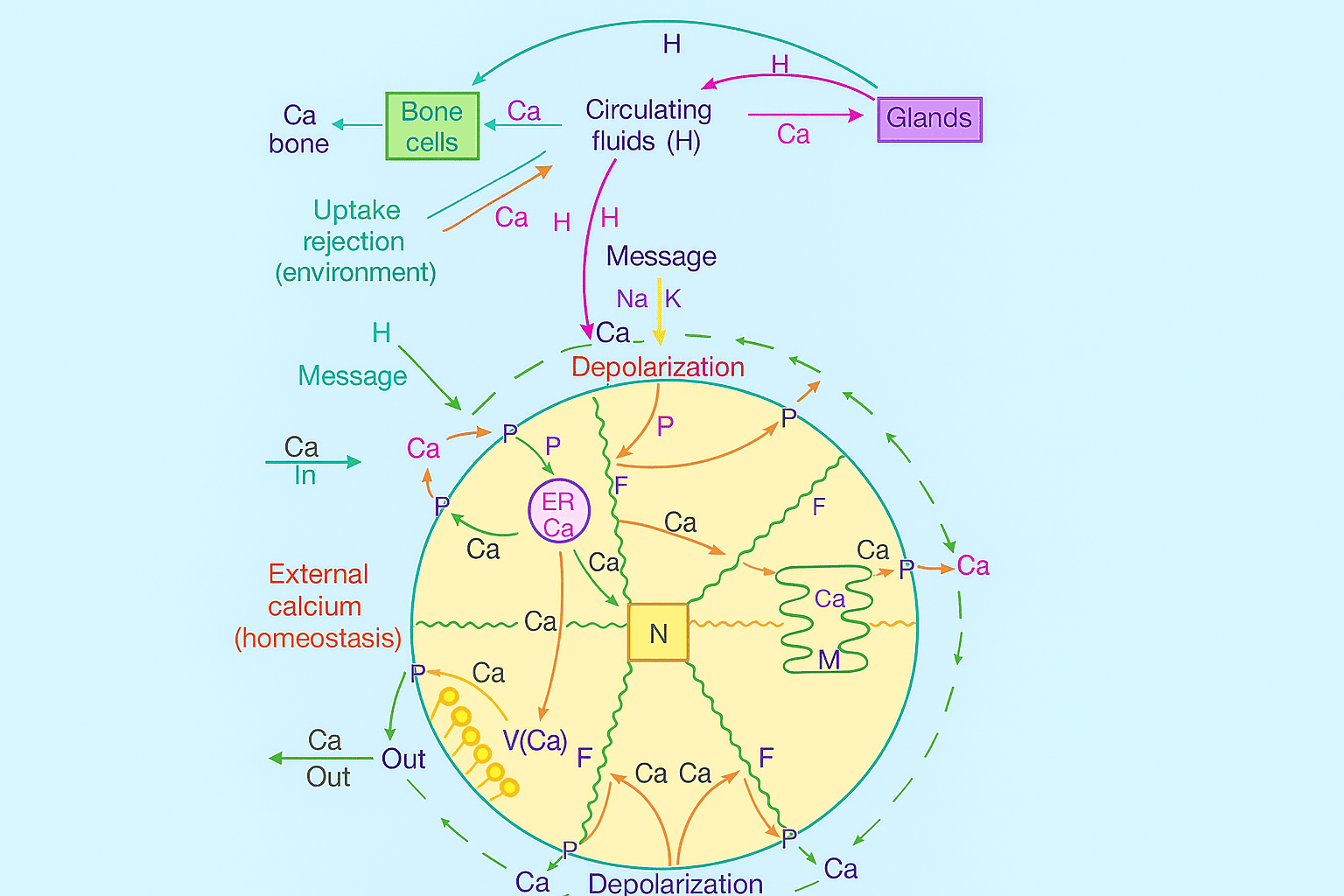
9. Ion Selectivity and Intracellular Calcium Regulation
9.1 Cytoplasmic Calcium Avoidance:
9.2 Magnesium vs. Calcium:
9.3 Selective Binding Examples:
9.4 Messenger Properties of Calcium:
1. Tightly Controlled Gradients:
2. Fast Reaction Rates:
10. Evolutionary Adaptation and Functional Mastery of Calcium Ions
10.1 Primitive Functions:
10.2 Eukaryotic Evolution:
10.3 Messenger Ion Development:
10.4 Expansion of Calcium Functions in Higher Organisms:
10.5 Intracellular and Intercellular Communication:
10.6 Buffering and Storage:
11. Conclusion
13. FAQs
1. What is the biological importance of calcium in the human body?
2. How does calcium act as a cellular messenger?
3. What is the role of calcium in muscle building and bodybuilding?
4. Why is calcium regulation important in living organisms?
5. How is calcium involved in both structure and signaling within the body?
Recent Posts
Sequence Alignment plays a vital role in the subsequent analysis of NGS data, where millions of sequenced DNA fragments (reads) need to be aligned with a chosen reference sequence in a timely manner.
FASTQ files serve as the “raw data files” for any sequencing application, indicating that they are “unaltered.” Consequently, this file format is utilized for performing Quality Checks on sequencing reads. The Quality Check process is typically carried out using the FastQC tool developed by Simon Andrews from Babraham Bioinformatics.
FASTA format is a text-oriented format utilized for depicting either nucleotide sequences or peptide sequences, where nucleotides or amino acids are denoted by a single-letter code.
Mammalian expression systems enable the production of complex, functional recombinant proteins with proper folding and post-translational modifications. These systems are ideal for studying human proteins in a near-native environment, offering advantages in scalability, gene delivery, and purification. HEK293 and CHO cells remain the most widely used hosts, supporting both transient and stable expression strategies for academic and pharmaceutical applications.
Gas Chromatography (GC) stands as one of the most powerful and versatile analytical techniques used to separate and analyze compounds in complex mixtures. At its core, GC enables the identification and quantification of chemical substances based on their molecular composition and retention behaviors during migration through a chromatographic column.