Biotinylation of Proteins: A Deep Dive Clinical Uses
Biotinylation is the process by which biotin—a vital water-soluble B-vitamin—is covalently attached to proteins. This modification most notably occurs in a specific class of enzymes called carboxylases, which are essential for several key metabolic pathways, including amino acid metabolism, fatty acid synthesis, and gluconeogenesis. In biological systems, each carboxylase is typically biotinylated at a single lysine residue, a reaction catalyzed by biotin:protein ligase (BPL). This highly conserved enzymatic process is found across all domains of life—bacteria, plants, and animals—underlining its fundamental biological importance. In addition to naturally occurring enzymatic biotinylation, proteins can also be biotinylated artificially via in vitro chemical synthesis. In this context, biotin can be attached to any accessible reactive group on the protein, such as amines or thiols, thereby expanding its utility beyond biological systems. This synthetic flexibility makes biotinylation a powerful tool in biotechnology, protein purification, cell tracking, and diagnostic applications. Chemically biotinylated proteins are indispensable tools in cell biology, biochemistry, and clinical research. Their significance lies in the exceptionally strong and specific interaction between biotin (and its analogs) and avidin or streptavidin, which underpins a wide array of laboratory and medical applications. From a clinical standpoint, biotinylated proteins hold particular importance for two key reasons: Endogenous Deficiency – A failure to synthesize adequate levels of naturally biotinylated proteins can lead to severe metabolic disorders, particularly in newborns and young children. Cancer Diagnosis and Therapy – Biotinylated proteins are instrumental in the improved detection and targeted treatment of tumors. In analytical sciences, biotinylation is extensively used for: Protein detection and quantification, Affinity-based purification systems, Labeling and tracking proteins in live-cell studies. Biotinylated proteins found within cells are primarily biotin-dependent carboxylases such as acetyl CoA carboxylase, beta-methylcrotonyl CoA carboxylase, propionyl CoA carboxylase, and pyruvate carboxylase. These enzymes are compartmentalized within the cell—acetyl CoA carboxylase is cytosolic, whereas the others localize to the mitochondria. In bacteria, biotin is attached to a biotin carboxyl carrier protein, which serves as part of bacterial carboxylase complexes. The biotinylation process unfolds in two steps: activation of biotin by ATP to form biotinyl-5'-adenosine monophosphate, followed by its ligation to a specific lysine residue within the target protein via holocarboxylase synthetase (in eukaryotes) or biotin protein ligase (in prokaryotes). Cytosolic: Acetyl CoA carboxylase, Mitochondrial: β-Methylcrotonyl CoA carboxylase, Propionyl CoA carboxylase, Pyruvate carboxylase, Bacterial: Biotin carboxyl carrier protein (subunit of bacterial carboxylases). Step 1: Biotin + ATP → Biotinyl-AMP + Pyrophosphate, Step 2: Biotinyl group transferred to lysine in the biotin domain via HS (eukaryotes) or BPL (prokaryotes), This process is irreversible under physiological conditions. Composed of 67–85 amino acids Characterized by a β-sheet-rich fold (eight β-strands forming two antiparallel sheets) Conserved motif: Glu–Ala–Met–Lys–Met, with Met–Lys–Met being invariant High cross-species recognition between enzymes and substrates, Bacterial BPL can biotinylate mammalian domains and vice versa. Structural integrity of the biotin domain, Functional requirements for catalytic activity in the carboxylases. Biotinylated proteins are degraded to biocytin (biotin + lysine), Biotinidase cleaves biocytin to recycle free biotin and lysine This dual consideration—enzyme recognition and catalytic necessity—explains the evolutionary stability of both the biotin ligases and their targets, ensuring efficient metabolism and biotin recycling across species. Disorders caused by deficiencies in biotin-dependent carboxylases fall into two main categories, based on whether they respond to biotin therapy. The non-responsive forms arise from genetic mutations in the carboxylases themselves (OMIM: 200350, 210200, 232050, 232000, 266150), and these conditions typically cannot be treated with supplemental biotin. In contrast, the biotin-responsive forms result from genetic defects in either holocarboxylase synthetase (OMIM: 253270) or biotinidase (OMIM: 25360). Clinically, these two types can often be distinguished by the age of onset: holocarboxylase synthetase deficiency tends to manifest within the first three months of life, while biotinidase deficiency usually presents later. In cases of holocarboxylase deficiency, the mutations often increase the Michaelis constant (Km) for biotin, meaning that higher concentrations of biotin are needed for effective enzyme activity. Thus, high-dose biotin therapy can restore sufficient biotinylation of apocarboxylases. The success of biotin treatment in biotinidase deficiency highlights the importance of biotin recycling. Biotinidase is essential for cleaving biocytin (biotin attached to lysine) during protein degradation, thereby regenerating free biotin and maintaining carboxylase activity. Additionally, biotinidase plays a key role in clinical imaging and therapeutic strategies. It can degrade radiolabeled biotin derivatives, which are frequently used in conjunction with biotinylated antibodies to image or target tumors. The primary objective of protein biotinylation is to detect the altered protein using avidin, which is linked to a reporter group (such as a radionuclide, chromophore, fluorophore, or enzyme). The identification of biotinylated proteins has been crucial in Western blotting to assess the expression of proteins in various cells subjected to different stimuli or growth conditions, for the immunocytochemical and immunohistochemical localization of proteins within cellular compartments or tissues, and for the discovery and monitoring of surface-biotinylated proteins. Additionally, immobilized avidin (and its derivatives) can facilitate the affinity capture and purification of biotinylated proteins, or the capture of receptors using biotinylated ligands. Biotinylation of proteins can be accomplished through the covalent attachment of a biotin derivative to any reactive functional group on a protein. The most frequently utilized sites include primary amines (the α- or ε-amino groups of the N-terminal or lysyl residues, respectively), thiol groups (from cysteinyl residues), and carbohydrate groups. The reactive derivatives of biotin for these reactions include N-hydroxysuccinimide esters, iodoacetyl, disulfide derivatives of biotin, and hydrazide derivatives of biotin, respectively. A critical aspect of effective biotinylation of proteins is ensuring that avidin can bind to the biotinyl group post-conjugation. This necessitates adequate spacing between the surface of the biotinylated protein and avidin, as biotin is accommodated in a relatively deep pocket of avidin. Klaus Hofmann addressed this challenge by discovering that an ε-aminocaproyl group provides a long enough spacer for this purpose. The application of sulfo-N-hydroxysuccinimidyl esters (sulfo-NHS esters) for biotin derivatization, introduced by James Staros, has made the use of biotin derivatives even easier by enhancing the water solubility and amine specificity of the reactive ester. Notably, sulfo-NHS esters are unable to penetrate cell membranes, allowing for the targeted labeling of cell-surface proteins via biotinylation of their extracellular regions. Biotinylated antibodies have proven highly effective in the detection and treatment of tumors, owing to the strong and specific interaction between biotin and avidin or streptavidin. This method allows for precise targeting of tumor cells and enables both imaging and therapeutic interventions. Biotinylated antibodies are first introduced into the bloodstream, where they travel and bind specifically to tumor antigens. This localization is crucial for ensuring that subsequent steps act directly at the tumor site. After the antibodies have accumulated at the tumor, avidin or streptavidin is administered. These proteins bind to the biotin groups on the antibodies and still retain free biotin-binding sites for the next step. A radionuclide-labeled biotin derivative is then administered. It binds to the remaining free sites on the avidin or streptavidin, effectively delivering the radioactive payload directly to the tumor. Imaging of tumors, using techniques like immunoscintigraphy or PET scans. Targeted radiotherapy, by delivering therapeutic radionuclides to the tumor cells. However, it's important to note that biotinidase, a naturally occurring enzyme in the bloodstream, can degrade biotin derivatives like biocytin analogs. This can interfere with the effectiveness of biotin-based targeting strategies. Therefore, understanding and managing the biotin cycle is essential for maximizing clinical success. Biotinylation of proteins stands as a powerful and versatile technique in both basic research and clinical applications. From its natural role in enabling essential enzymatic functions through biotin-dependent carboxylases to its synthetic use in protein labeling, detection, and purification, biotinylation exemplifies the intersection of biochemistry and biotechnology. Understanding the biosynthesis, chemical strategies, and clinical significance of biotinylated proteins provides deeper insight into not only metabolic health but also the advancement of diagnostic and therapeutic tools—particularly in cancer imaging and treatment. As technologies evolve, the precise and targeted nature of biotin-avidin interactions continues to make biotinylation an indispensable asset across the life sciences. Naturally biotinylated proteins are primarily biotin-dependent carboxylases, which play key roles in essential metabolic processes. In mammalian cells, these include: Acetyl-CoA carboxylase (cytosolic), Propionyl-CoA carboxylase, Pyruvate carboxylase, and Beta-methylcrotonyl-CoA carboxylase (all mitochondrial). In bacteria, biotinylation occurs on the biotin carboxyl carrier protein, a subunit of bacterial carboxylases. Proteins can be chemically biotinylated by attaching biotin derivatives to reactive groups such as: Primary amines (e.g., lysine residues), Thiol groups (e.g., from cysteine), or Carbohydrate groups. Common reagents include N-hydroxysuccinimide (NHS) esters and sulfo-NHS esters, the latter of which is membrane-impermeable and ideal for labeling cell surface or membrane proteins. Yes. In both mammalian and E. coli cells, biotinylated proteins can be detected using avidin- or streptavidin-based methods. These include: Western blotting, Immunocytochemistry, and Affinity purification techniques. Because biotin binds avidin with high affinity, even low-abundance proteins can be efficiently identified and tracked. Purification is commonly achieved using affinity capture methods, where immobilized avidin or streptavidin is used to bind biotinylated proteins. This allows researchers to: Enrich target proteins, Pull down receptors using biotinylated ligands, and Isolate specific molecules for further analysis, such as mass spectrometry. Clinically, a deficiency in endogenous biotinylated proteins—due to genetic defects in carboxylases, holocarboxylase synthetase, or biotinidase—can result in serious metabolic disorders. Detecting these proteins aids in: Diagnosing conditions like multiple carboxylase deficiency, Monitoring biotin treatment efficacy, and Supporting tumor imaging, where biotinylated antibodies and radiolabeled biotin derivatives are used.
1. Introduction
2. Analytical and Clinical Applications of Biotinylated Proteins
3. Biosynthesis and Degradation of Endogenous Biotinylated Proteins
3.1 Key Highlights:
1. Biotin-Dependent Proteins:
2. Biotinylation Mechanism:
3. Biotin Domain Features:
4. Evolutionary Conservation:
5. Specificity Determinants:
6. Degradation:
4. Medical Significance of Biotinylated Proteins
5. Chemical Strategies and Applications in Protein Biotinylation
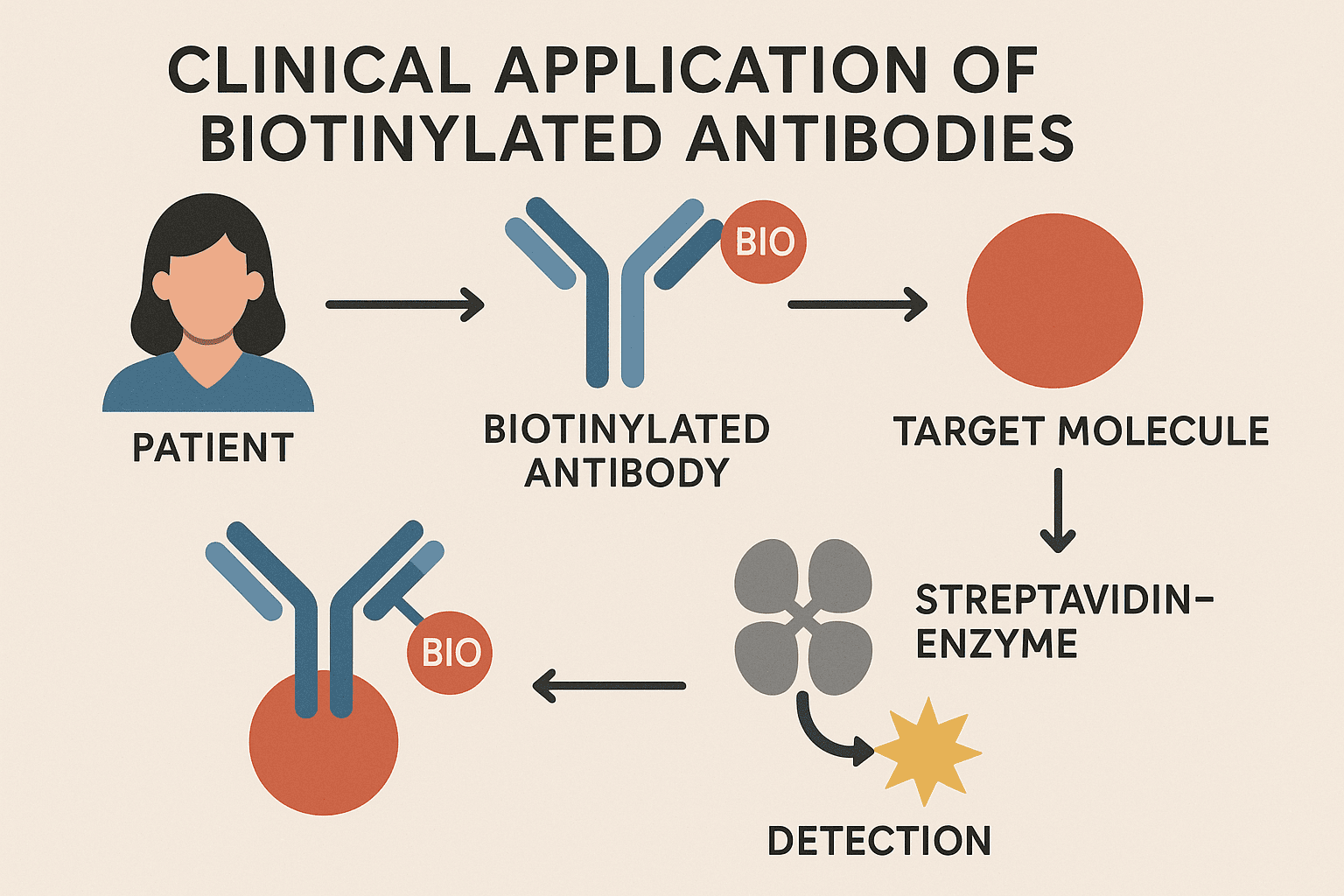
6. Clinical Use of Biotinylated Antibodies
6.1 The process involves several key steps:
1. Tumor Targeting:
2. Avidin/Streptavidin Administration:
4. Radionuclide Delivery:
This method enables:
7. Conclusion
8. FAQs
1. What are naturally biotinylated proteins and where are they found?
2. How are proteins chemically biotinylated for research purposes?
3. Can biotinylated proteins be detected in mammalian or E. coli cells?
4. How are biotinylated proteins purified in the lab?
5. What is the clinical relevance of detecting endogenous biotinylated proteins?
Recent Posts
Sequence Alignment plays a vital role in the subsequent analysis of NGS data, where millions of sequenced DNA fragments (reads) need to be aligned with a chosen reference sequence in a timely manner.
FASTQ files serve as the “raw data files” for any sequencing application, indicating that they are “unaltered.” Consequently, this file format is utilized for performing Quality Checks on sequencing reads. The Quality Check process is typically carried out using the FastQC tool developed by Simon Andrews from Babraham Bioinformatics.
FASTA format is a text-oriented format utilized for depicting either nucleotide sequences or peptide sequences, where nucleotides or amino acids are denoted by a single-letter code.
Mammalian expression systems enable the production of complex, functional recombinant proteins with proper folding and post-translational modifications. These systems are ideal for studying human proteins in a near-native environment, offering advantages in scalability, gene delivery, and purification. HEK293 and CHO cells remain the most widely used hosts, supporting both transient and stable expression strategies for academic and pharmaceutical applications.
Gas Chromatography (GC) stands as one of the most powerful and versatile analytical techniques used to separate and analyze compounds in complex mixtures. At its core, GC enables the identification and quantification of chemical substances based on their molecular composition and retention behaviors during migration through a chromatographic column.