Calbindin: A Key Player Among Calcium-Buffering Proteins
Calbindins—particularly calbindin-D9k and calbindin-D28k—are crucial members of a large family of high-affinity calcium-binding proteins. These proteins are characterized by the EF-hand structural motif, a conserved helix-loop-helix configuration that forms a specialized binding site for calcium ions. This motif, shared by over 300 related proteins, allows calbindins to play a central role in intracellular calcium buffering. Among these, calbindin-D28k was the first protein identified as a downstream target of vitamin D. It has a molecular weight of approximately 28 kDa, contains four high-affinity calcium-binding sites, and is predominantly expressed in the intestines of birds, as well as in the kidneys, brains, and pancreases of both birds and mammals. Notably, calbindin-D28k is evolutionarily conserved, reflecting its fundamental biological role. In contrast, calbindin-D9k, a smaller protein of about 9 kDa, harbors two calcium-binding domains. It is primarily found in the intestines of mammals and shows no sequence homology with calbindin-D28k. Unlike its counterpart, calbindin-D9k is not evolutionarily conserved, being unique to mammals. This review explores the structure, distribution, functional roles, and regulatory mechanisms of calbindins, along with insights from calbindin knockout models that have expanded our understanding of their physiological significance. Calbindin-D28k, originally known as the vitamin D-dependent calcium-binding protein (CaBP), was first identified in the intestines of birds by Wasserman and Taylor in 1966. It was officially renamed calbindin-D28k in 1985. Studies of its tissue distribution and its colocalization with the vitamin D receptor (VDR) have significantly advanced the understanding of the vitamin D endocrine system. Both chicken and mammalian calbindin-D28k are single-chain polypeptides made up of 261 amino acids. They have a molecular weight of approximately 28 kDa, as measured by SDS-PAGE. The proteins feature blocking groups at the amino terminus, contributing to their stability. Calbindin-D28k shows remarkable evolutionary conservation: 98% homology among mammals, 79% homology between mammals and chickens, This high degree of similarity reflects its essential physiological role in calcium-regulated intracellular processes. Calbindin-D28k is an acidic and heat-stable member of the EF-hand protein family. It binds calcium ions with high affinity (Kd ≈ 10⁻⁸ to 10⁻⁶ M), Each EF-hand domain consists of a 29–30 amino acid structure, including two α-helices connected by a 12-residue calcium-binding loop, The loop contains side chain oxygens that coordinate calcium binding, Out of six EF-hand domains, only four actively bind calcium. The human CALB1 gene, which encodes calbindin-D28k: Is located on chromosome 8, Spans 24 kilobases, Contains 11 exons. Since its first discovery in avian intestines, calbindin-D28k has also been found in a variety of tissues beyond those involved in calcium regulation. These include the kidney and bone, as well as other organs like the pancreas, testes, and brain. This widespread expression suggests a broader functional role in cellular calcium buffering, neuroprotection, and endocrine signaling. Calbindin-D28k in the avian intestine, along with calbindin-D9k in the mammalian intestine, is strongly induced by 1,25(OH)₂D₃. It is located in the cytoplasm of absorptive cells, which supports its suggested role in intestinal calcium absorption (see also the section on calbindin-D9k). Calbindin-D28k shows a significant increase in expression in response to 1,25(OH)₂D₃ stimulation. It is localized in the cytoplasm of absorptive intestinal cells, which aligns with its proposed function in transporting calcium. Calbindin may also play a protective role by buffering calcium levels, which helps to prevent calcium-induced cell death during vitamin D-mediated calcium transport in the intestine. This dual role—facilitating absorption and protecting cells—underscores calbindin-D28k’s importance in maintaining intestinal calcium homeostasis during high-demand states. In mammals, chickens, and reptiles, renal calbindin-D28k is found primarily in the cytoplasm, and to a lesser extent in the nucleus, of distal convoluted tubule and connecting tubule cells in the distal nephron. The vitamin D receptor (VDR) is also mainly located in this region. In the distal nephron, 1,25(OH)₂D₃ enhances parathyroid hormone (PTH)-mediated calcium uptake at the apical membrane of distal tubule cells. Calbindin-D28k is induced by 1,25(OH)₂D₃, although its precise role in vitamin D-regulated calcium transport in the distal nephron remains unclear. Similar to its function in the intestine, renal calcium transport involves calcium entering through apical calcium channels, diffusing through the cytoplasm, and being actively transported out via a basolateral calcium ATPase. Renal calbindin-D28k may assist in moving calcium across the cell to the basolateral membrane, while also buffering intracellular calcium and preventing calcium-induced cytotoxicity. The TRPV5 channel, located on the apical membrane, controls calcium entry during active reabsorption and is co-expressed with calbindin-D28k in the distal nephron. Both TRPV5 and calbindin-D28k are regulated by 1,25(OH)₂D₃ and dietary calcium levels. Under low intracellular calcium conditions, calbindin-D28k interacts directly with TRPV5, influencing the activity of the epithelial calcium channel. These findings indicate that calbindin-D28k in the kidney may also act as a modulator of calcium influx, in addition to its buffering and transport functions. Calbindin-D28k has been found in human pancreatic islet cells, chick insulin-secreting beta cells, and in both alpha and beta cells of the rat pancreas. It has been suggested that calbindin-D28k may influence insulin release triggered by membrane depolarization by regulating intracellular calcium (Ca²⁺) levels. Calbindin-D28k, acting as a calcium buffer, helps to prevent mitochondrial damage caused by excessive intracellular Ca²⁺ and the associated production of free radicals. Through this buffering action, calbindin-D28k offers protection against cytokine-induced destruction of pancreatic beta cells. These findings are important for type 1 diabetes research, suggesting a possible role for calbindin-D28k in preventing autoimmune-mediated beta-cell damage. Calbindin-D28k also regulates calcium entry in beta cells via L-type voltage-dependent calcium channels. In rat beta cells, the alpha₁ subunit of the L-type calcium channel has been shown to associate with calbindin-D28k, influencing the channel's sensitivity. When calbindin-D28k is present, L-type channels become more sensitive to calcium-dependent inactivation, indicating a modulatory role. Altogether, these observations highlight that pancreatic calbindin-D28k is important both for maintaining intracellular calcium homeostasis and for modulating calcium influx in insulin-secreting cells. Calbindin-D28k has been detected in spermatogonia and spermatocytes within the seminiferous tubules, as well as in some interstitial Leydig cells of the testes in both chicks and rats. Its presence in these cells suggests a possible role in spermatogenesis. Calbindin-D28k is also present in the tubular gland cells of the chick shell gland, which are responsible for calcium secretion during eggshell formation. In female mice, but not in rats, calbindin-D28k is found in several reproductive tissues, including the endometrium, glandular epithelium of the uterus, oviduct epithelium, and primary ovarian follicles. The expression of calbindin-D28k in these reproductive tissues is not regulated by 1,25(OH)₂D₃. Instead, estradiol has been shown to regulate calbindin-D28k levels in the uterus. Although the exact function of uterine calbindin-D28k remains uncertain, it is thought to play a role in facilitating transcellular calcium transport in uterine epithelial cells. Calbindin-D28k is widely distributed in the nervous systems of mammals, birds, reptiles, amphibians, fish, and mollusks, functioning independently of vitamin D. It is found in many neuronal populations and some epithelial cells of the ventricular system. In the brain, it plays an essential role in calcium buffering, neuroprotection, and the modulation of synaptic activity. Neurons containing calbindin-D28k are present in regions such as the cerebral cortex (layers 2–4), hippocampus, cerebellum (especially Purkinje cells), hypothalamus, amygdala, and thalamus. It is also expressed in sensory neurons of the visual and auditory systems, including photoreceptors, cochlear and vestibular hair cells, and pinealocytes. Calbindin-D28k buffers intracellular calcium, helping to prevent calcium-induced neurotoxicity and regulate neuronal excitability and synaptic transmission. Experimental overexpression of calbindin-D28k has been shown to alter calcium signaling, affecting synaptic plasticity and neuronal firing patterns. In the suprachiasmatic nucleus (SCN) of mice, calbindin-D28k is involved in the regulation of circadian rhythms. Its absence leads to disrupted circadian locomotor patterns, although acute light responses remain unaffected. These findings underscore the critical role of calbindin-D28k in maintaining neuronal health and circadian rhythm stability. 1,25-Dihydroxyvitamin D₃ [1,25(OH)₂D₃] is known to stimulate calbindin-D28k expression in the intestines and kidneys of birds, as well as in the kidneys of mammals. However, the regulatory response is more complex and limited than traditionally expected. Studies have shown that the promoters of calbindin-D28k in both chicken and mouse models exhibit only modest transcriptional activation in response to 1,25(OH)₂D₃. The production of calbindin-D28k mRNA occurs primarily after a delay following exposure to 1,25(OH)₂D₃, suggesting that posttranscriptional mechanisms play a dominant role in its regulation. These findings highlight that the action of 1,25(OH)₂D₃ on calbindin-D28k does not fit entirely within the standard hormone-receptor transcriptional model, and instead involves more complex regulatory pathways. This complexity underscores the need for a broader regulatory framework to fully understand how 1,25(OH)₂D₃ controls calbindin-D28k expression. Calbindin-D28k expression is influenced not only by 1,25(OH)₂D₃ but also by other hormones, dietary elements, and neurotrophic factors, depending on the tissue type and species. Glucocorticoids reduce calbindin-D28k expression in the intestine of vitamin D-treated chicks, along with decreasing calcium absorption. Estradiol regulates calbindin-D28k in a species-specific manner—it increases expression in the avian shell gland but decreases it in the uterus of female mice. Gender-specific regulation is evident in mice: males show lower renal calbindin-D28k levels and greater urinary calcium loss compared to females. Androgen deficiency increases expression, which is suppressed by testosterone. In the nervous system, calbindin-D28k expression is independent of 1,25(OH)₂D₃. Instead, it is stimulated by factors such as TNFs, BDNF, NT-3, and FGF, which help protect neurons from excitotoxic damage. Additional regulators include corticosterone (induces expression in rat hippocampus), retinoic acid (in neuronal tumor cells), and IGF-I and insulin, which activate calbindin-D28k in neuronal cells and Purkinje cells. These findings highlight that calbindin-D28k regulation is highly complex and tissue-specific, extending beyond the classical vitamin D pathway. Calbindin-D28k is an evolutionarily conserved protein, underscoring its essential role in intracellular calcium-dependent processes. It is regulated by 1,25(OH)₂D₃ in specific tissues like the avian intestine and kidneys of both birds and mammals. Additionally, calbindin-D28k is expressed in various non-renal and non-intestinal tissues, where its regulation occurs through mechanisms independent of 1,25(OH)₂D₃. The evolutionary conservation of calbindin-D28k highlights its biological importance across species. In the intestine and kidneys, its expression is stimulated by 1,25(OH)₂D₃, reinforcing its role in vitamin D-mediated calcium regulation. Calbindin-D28k is also found in other tissues such as the brain, pancreas, and uterus, where it is regulated by alternative factors. One of its key protective functions is to buffer intracellular calcium, thereby preventing apoptotic cell death through the inhibition of caspase-3. It also contributes to maintaining intracellular calcium homeostasis and modulating calcium entry into the cell. In 1967, Kallfelz, Taylor, and Wasserman identified a vitamin D-regulated calcium-binding protein with a molecular weight of approximately 9000 in the intestines of rats. This protein, later named calbindin-D9k, is a cytosolic calcium-binding protein found exclusively in mammals. Calbindin-D9k is composed of 79 amino acid residues, has a molecular weight of 9016, and is both acidic and heat-stable. The human calbindin-D9k sequence shares 78% homology with rat and 74% with mouse, indicating moderate evolutionary conservation within mammals. It contains two EF-hand domains, made up of 29 and 31 amino acids, with each domain capable of binding one calcium ion. Calbindin-D9k is a member of the S100 family of calcium-binding proteins. The CALB3 gene, which encodes calbindin-D9k, spans approximately 5.5 kb, includes three exons, and is located on chromosome Xp22.2 in humans. This protein is primarily localized in the intestines of mammals, especially involved in calcium absorption. Lower levels of calbindin-D9k are also found in the kidneys of mice and neonatal rats, specifically in the distal nephron. It is additionally expressed in the placenta (rats and mice), uterus and lungs (rats), and in ameloblasts and osteoblasts associated with rodent teeth. Like calbindin-D28k in avian intestines, calbindin-D9k in the mammalian intestine is strongly induced by 1,25(OH)₂D₃ and has been proposed to assist in the intracellular diffusion of calcium toward the basolateral membrane. However, studies using calbindin-D9k knockout mice have challenged this view. Research shows that active intestinal calcium transport still occurs effectively in calbindin-D9k knockout mice, even after 1,25(OH)₂D₃ treatment or under a low-calcium diet. Serum calcium levels remain normal in calbindin-D9k knockout mice, regardless of age or sex, indicating that its absence does not disrupt systemic calcium homeostasis. These findings suggest that other mechanisms or proteins may compensate for the lack of calbindin-D9k in maintaining calcium absorption. It is also proposed that calbindin-D9k might have alternative roles, such as modulating TPRV6 activity—a vitamin D-responsive calcium channel—or buffering intracellular calcium to prevent toxic accumulation. Calbindin-D9k is expressed in the placenta of rats and mice, and in the endometrium and myometrium of rats. Its presence in these reproductive tissues suggests possible roles in maternal-fetal calcium transport and uterine function. Calbindin-D9k levels in the placenta increase near the end of gestation, when the fetal demand for calcium is highest, supporting its potential role in calcium transfer from mother to fetus. Its detection in the myometrium suggests that calbindin-D9k may help regulate intracellular calcium, which could affect the strength and timing of uterine contractions. Despite these proposed roles, calbindin-D9k knockout mice reproduce normally, and their offspring are indistinguishable from wild-type pups, suggesting functional compensation by other factors. The expression of intestinal calbindin-D9k is regulated by 1,25(OH)₂D₃, showing an initial transcriptional response followed by posttranscriptional effects, similar to calbindin-D28k in birds. Other key factors such as Cdx-2 and hormones like glucocorticoids and estradiol also play significant roles in its regulation, depending on the tissue type. Cdx-2, an intestine-specific transcription factor, is essential for the transcriptional activation of the calbindin-D9k gene, in addition to the involvement of the vitamin D receptor (VDR). Glucocorticoid treatment in mice leads to a decrease in duodenal calbindin-D9k mRNA expression, which correlates with reduced intestinal calcium absorption. It remains unclear whether this glucocorticoid-mediated reduction is due to a direct effect on the calbindin-D9k gene or through an indirect pathway. In the uterus of rats, estradiol directly stimulates calbindin-D9k expression, as confirmed by the presence of an estrogen-responsive element in its gene promoter. 1,25(OH)₂D₃ does not influence uterine calbindin-D9k expression, highlighting a tissue-specific regulation pattern. Calbindin-D9k is a mammal-specific protein that differs structurally and functionally from calbindin-D28k. It belongs to the S100 family of calcium-binding proteins and plays a role in calcium regulation, particularly in the intestine and kidney, where it is regulated by 1,25(OH)₂D₃. Though its presence in other organs has been noted, its tissue distribution is more restricted than that of calbindin-D28k. Calbindin-D9k shares no sequence homology with calbindin-D28k, despite both being calcium-binding proteins. It is primarily regulated by 1,25(OH)₂D₃ in the intestinal and renal tissues of mammals. Compared to calbindin-D28k, calbindin-D9k has a more limited distribution, found in fewer organs such as the uterus, placenta, and bone. Studies using calbindin-D9k knockout mice suggest that its absence does not impair calcium homeostasis, implying that other compensatory mechanisms or factors may substitute for its function. Calbindin-D28k and calbindin-D9k should no longer be viewed exclusively as vitamin D-dependent proteins. Their regulation is far more complex, involving multiple hormonal and molecular pathways beyond 1,25(OH)₂D₃. These calcium-binding proteins serve diverse, tissue-specific functions that extend beyond the traditional boundaries of the vitamin D endocrine system. Both calbindin-D28k and calbindin-D9k are expressed in a variety of tissues and species, reflecting their widespread physiological importance. Their roles include maintaining intracellular calcium homeostasis, modulating calcium influx, and protecting cells from calcium-induced damage. The regulatory mechanisms controlling calbindin expression involve vitamin D, estradiol, glucocorticoids, and neurotrophic factors, demonstrating their multi-level hormonal and functional integration. Continued research into calbindins will be essential to fully understand their non-classical roles, especially in neuroprotection, reproduction, and cellular signaling. Calbindin functions primarily as a calcium-buffering protein. It binds calcium ions within cells, helping to regulate intracellular calcium levels, facilitate calcium transport, and prevent calcium-induced cell damage. Calbindin-D9k and calbindin-D28k differ in size, structure, and distribution. Calbindin-D9k is a smaller protein found only in mammals and belongs to the S100 protein family, while calbindin-D28k is evolutionarily conserved across species and is part of the EF-hand calcium-binding family. They are not homologous and have distinct gene origins. No, while calbindins were initially identified as vitamin D-dependent, their regulation is more complex. 1,25(OH)₂D₃ influences their expression in some tissues, but other factors like estradiol, glucocorticoids, Cdx-2, and neurotrophic signals also play significant roles in tissue-specific regulation. In the intestine, calbindins (particularly D9k in mammals and D28k in birds) are believed to help transport calcium across epithelial cells and act as buffers to prevent calcium overload. However, studies in knockout mice suggest redundancy or compensation by other proteins, meaning calbindins may not be essential but still support calcium transport efficiency. In the brain, calbindin-D28k helps maintain calcium homeostasis, protect neurons from excitotoxicity, and influence synaptic activity. It is also expressed in reproductive tissues, pancreas, and kidneys, performing roles like modulating hormone release, supporting calcium reabsorption, and maintaining cell function under varying physiological demands.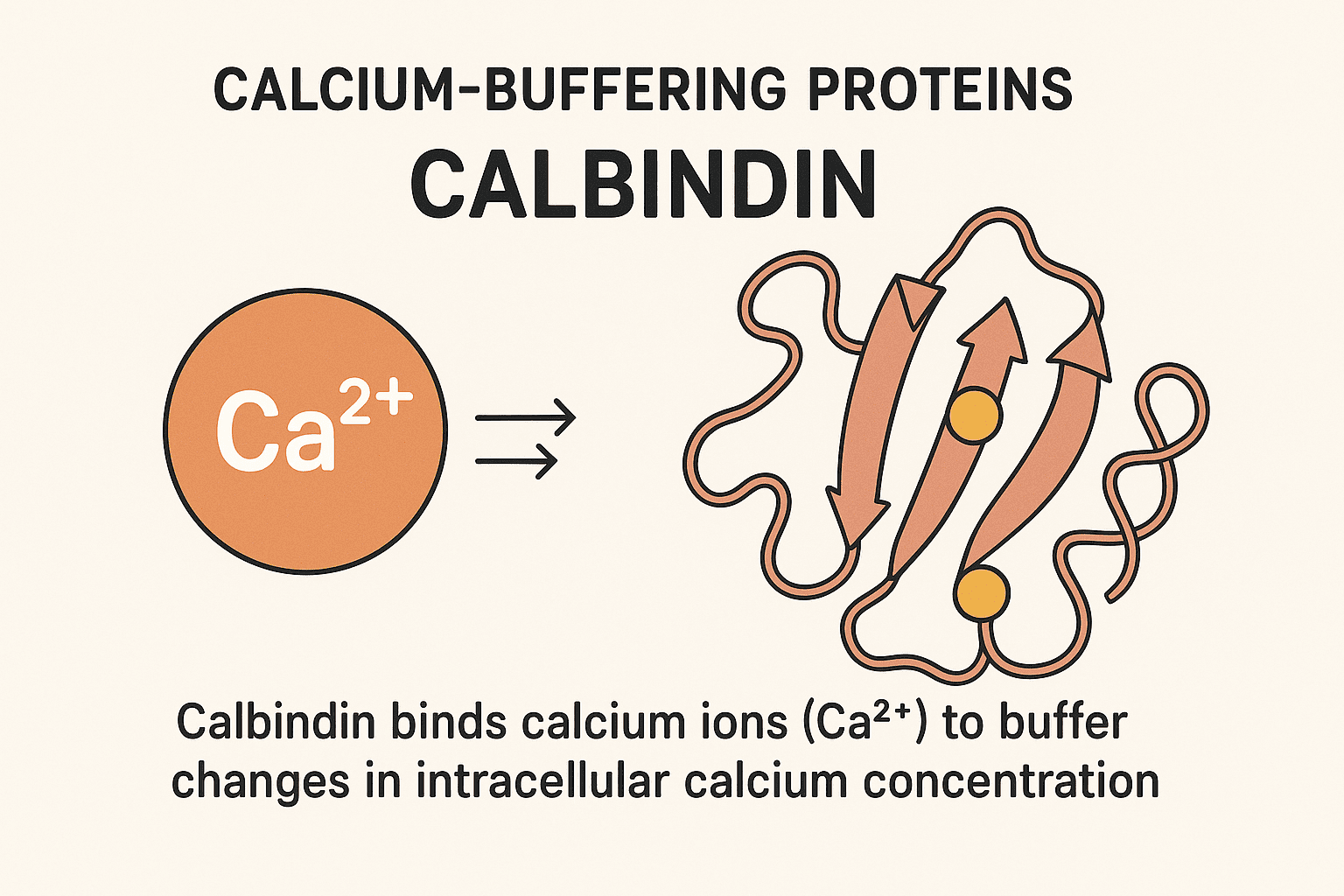
1. Introduction
2. d-D28k: Characterization and Tissue Distribution
2.1 Structure and composition:
2.2 Evolutionary conservation:
2.3 Biochemical characteristics:
2.4 Genetic information:
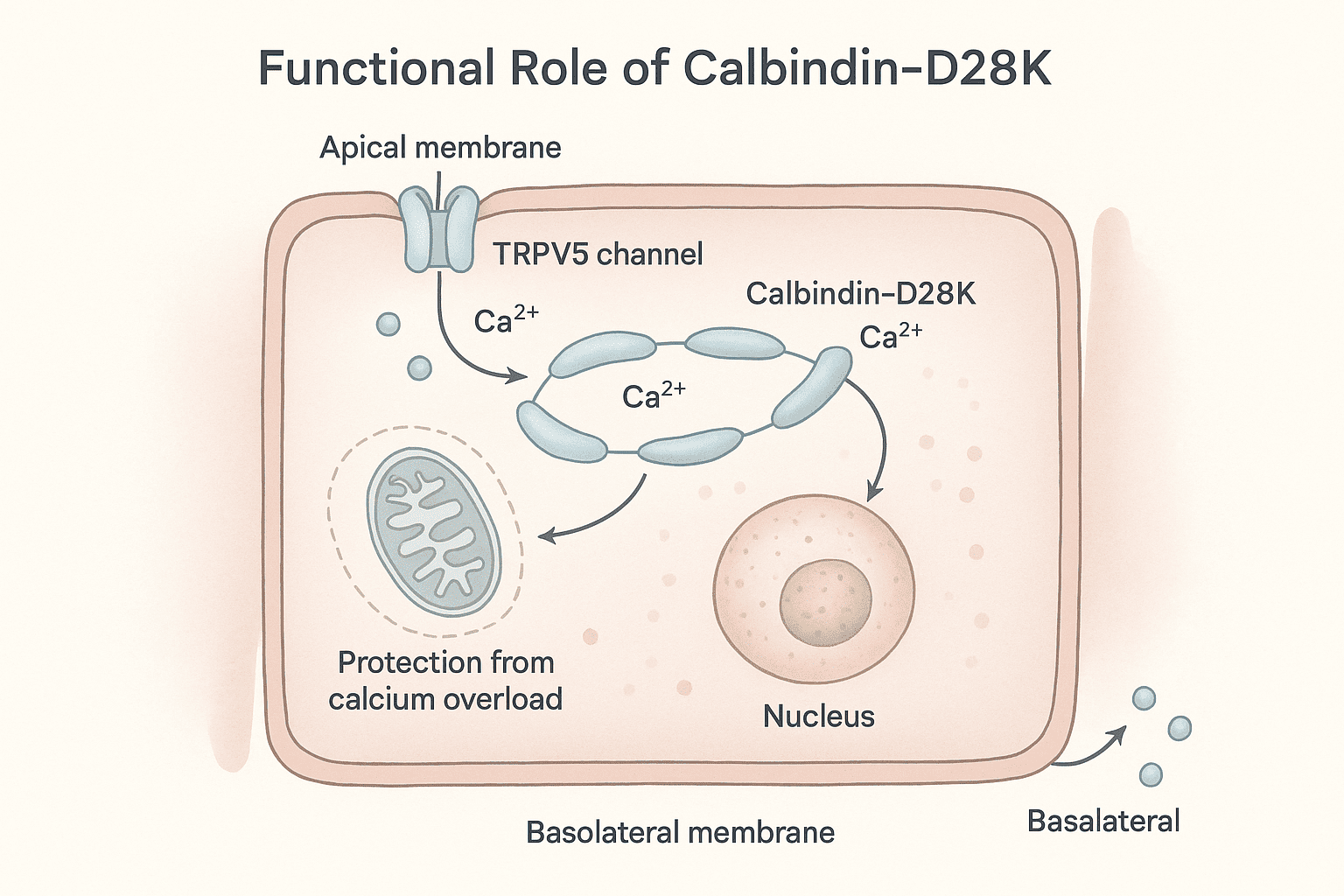
3. The Proposed Functional Significance of Calbindin-D28K
3.1 Intestine
3.2 Kidney
3.3 Pancreas
3.4 Reproductive Tissues
3.5 Nervous Tissue
4. Understanding the Regulation of Calbindin-D28K
4.1 Regulation by 1,25(OH)₂D₃
4.2 Hormonal and Molecular Modulation of Calbindin-D28k
4.3 Calbindin-D28k Summary
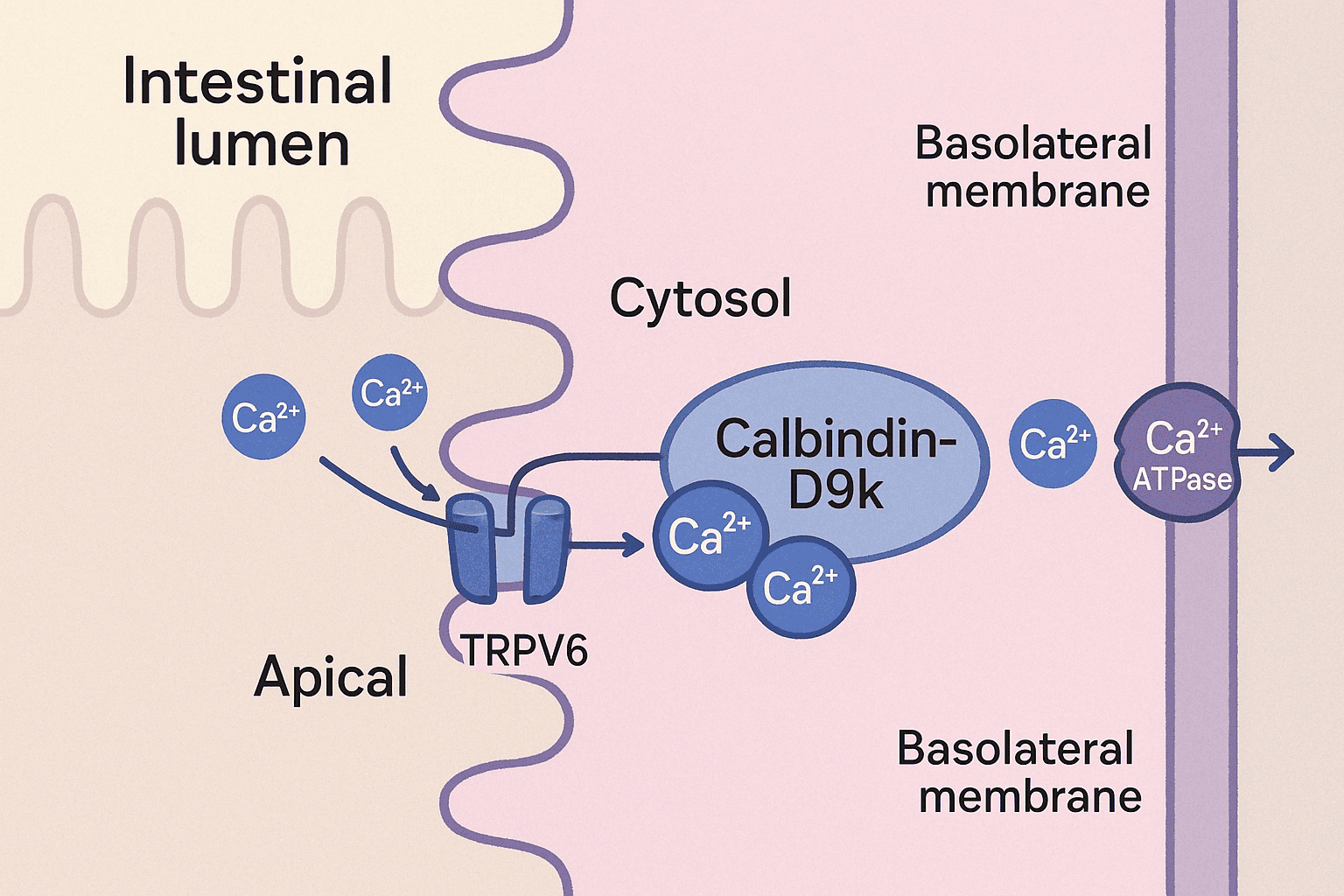
5. Calbindin-D9k: Characterization and Tissue Distribution
6. The Proposed Functional Significance of Calbindin-D9K
6.1 Intestinal and calbindin-D9k knockout mice
6.2 Reproductive Tissues
6.3 Regulation of Calbindin-D9k
6.4 Calbindin-D9k Overview
7. Conclusion
8. FAQs
1. What is the main function of calbindin?
2. What is the difference between calbindin-D9k and calbindin-D28k?
3. Is calbindin regulated only by vitamin D?
4. How is calbindin related to calcium absorption in the intestine?
5. What role does calbindin play in the brain and other tissues?
Recent Posts
Sequence Alignment plays a vital role in the subsequent analysis of NGS data, where millions of sequenced DNA fragments (reads) need to be aligned with a chosen reference sequence in a timely manner.
FASTQ files serve as the “raw data files” for any sequencing application, indicating that they are “unaltered.” Consequently, this file format is utilized for performing Quality Checks on sequencing reads. The Quality Check process is typically carried out using the FastQC tool developed by Simon Andrews from Babraham Bioinformatics.
FASTA format is a text-oriented format utilized for depicting either nucleotide sequences or peptide sequences, where nucleotides or amino acids are denoted by a single-letter code.
Mammalian expression systems enable the production of complex, functional recombinant proteins with proper folding and post-translational modifications. These systems are ideal for studying human proteins in a near-native environment, offering advantages in scalability, gene delivery, and purification. HEK293 and CHO cells remain the most widely used hosts, supporting both transient and stable expression strategies for academic and pharmaceutical applications.
Gas Chromatography (GC) stands as one of the most powerful and versatile analytical techniques used to separate and analyze compounds in complex mixtures. At its core, GC enables the identification and quantification of chemical substances based on their molecular composition and retention behaviors during migration through a chromatographic column.