Aminopeptidases: Functions,Properties &Structural Insights
Aminopeptidases (AP) facilitate the breakdown of amino acid residues from the amino end of peptide substrates. These enzymes typically exhibit a wide range of specificity, exist in various forms, and are found extensively across the plant and animal kingdoms. More than 100 APs have been isolated and/or examined, with over 50 genes having been cloned and analyzed. Different variants of these enzymes have been discovered in numerous tissues or cells, on cell surfaces, as well as in soluble cytoplasmic or secreted forms in both plants and animals. In certain cells, they represent a significant portion of the enzyme protein. Multiple physiological roles of APs have been recognized. Aminopeptidases play a crucial role in protein maturation, the breakdown of both non-hormonal and hormonal peptides, and potentially in the regulation of protein stability. Numerous disease conditions are linked to compromised proteolytic activity. Furthermore, several APs are known to catalyze reactions beyond peptide hydrolysis. Recent findings on these topics will be detailed below and in the related chapters. Aminopeptidases are present in both soluble and membrane-bound forms, distributed throughout various cellular compartments as well as in extracellular environments. Most aminopeptidases function as metalloenzymes, requiring at least one metal cofactor—most commonly zinc (Zn²⁺)—for catalytic activity. However, other metal ions such as iron (Fe²⁺), manganese (Mn²⁺), and cobalt (Co²⁺) may also serve as cofactors, and some enzymes incorporate one or two metal ions depending on their subclass. In certain cases, the physiologically relevant metal ion has not yet been definitively identified. Some specialized aminopeptidases, such as dipeptidyl- and tripeptidyl-peptidases, are not metalloenzymes but instead act as serine or cysteine proteases, expanding the enzymatic diversity within this group. Functionally, these enzymes may act in a processive manner—continuously cleaving residues from the N-terminus of a peptide until an unfavorable residue halts further action—or in a non-processive fashion, displaying high specificity for a particular amino acid and stopping after a single cleavage event. Aminopeptidases perform essential physiological roles that can be broadly classified into three main categories: processing/maturation, activation, and degradation. Enzymes responsible for processing and activation are typically highly specific and nonprocessive, targeting the N-terminal ends of proteins or peptides either co-translationally or post-translationally. These modifications often regulate the biological activity of the target molecules or prepare them for further functional processing. In contrast, degradative roles—which may include peptide inactivation—can involve either specific enzymes (especially for bioactive peptides) or more nonspecific enzymes involved in protein turnover, where they break down peptides into amino acids after proteasomal or extracellular cleavage. Modify the N-terminus of peptides or proteins, Occur during or after protein synthesis, Highly specific and nonprocessive. Trigger biological activity of precursor proteins/peptides, Enable further post-translational modifications. Break down small peptides into amino acids, May involve non specific enzymes for protein turnover, Includes extracellular peptide degradation or clearance of proteasomal fragments. Aminopeptidases play a crucial and highly conserved role in N-terminal cotranslational processing, a process that modifies nascent proteins as they are synthesized. Although protein biosynthesis universally begins with methionine (or N-formylmethionine in prokaryotes), most mature proteins do not retain this initial amino acid. This discrepancy is due to the coordinated action of signal peptidases and methionine aminopeptidases (MetAPs). Signal peptidases, which are endopeptidases, remove entire signal peptides—usually 20–25 amino acids long—from proteins entering the endoplasmic reticulum, mitochondria, or chloroplasts. MetAPs, a subclass of aminopeptidases from clan MG of metallo-peptidases, cleave off the initiating methionine from intracellular proteins. This processing is essential for cell viability across all domains of life. Nearly all organisms have at least one form of MetAP, reflecting its evolutionary importance. Methionine is scarce: Recycling it is vital to prevent a methionine-starved, potentially lethal state. Post-translational modifications: The exposed α-amino group (after methionine removal) often undergoes essential chemical modifications. Protein function: Some proteins require the penultimate residue—revealed after methionine cleavage—for proper folding or activity. Proteolytic protection: Retention of methionine at the N-terminus may shield proteins from degradation via the N-end rule pathway. Thus, methionine removal is a regulated and selective process that ensures protein function, stability, and cellular homeostasis. Methionine aminopeptidases (MetAPs) exhibit a strikingly conserved substrate specificity across all domains of life, reflecting their ancient and indispensable role in protein maturation. Despite species-specific structural differences, MetAPs universally target the initiator methionine for removal—but only when it is followed by certain small amino acids. This preference ensures efficient N-terminal processing and contributes significantly to protein function and cellular homeostasis. MetAPs are selective enzymes that recognize the context of the N-terminal sequence, particularly the identity of the penultimate residue, to determine whether methionine removal should occur. In Saccharomyces cerevisiae (yeast), while over half of the open reading frames (ORFs) are predicted to encode proteins with suitable N-terminal motifs, the actual proportion of processed proteins by mass may be even higher due to protein trafficking and signal peptide cleavage. MetAPs remove methionine only if followed by one of the seven smallest amino acids: Glycine (G), Alanine (A), Serine (S), Threonine (T), Cysteine (C), Proline (P), Valine (V). ~50% of ORFs are predicted to encode MetAP-cleavable N-termini. Up to 80% of soluble proteins (by mass) likely undergo MetAP-mediated N-terminal processing. Secretory and membrane proteins, as well as those imported into mitochondria and chloroplasts, typically have their N-termini cleaved during signal peptide removal, contributing further to methionine recycling. The cleaved signal peptides are degraded completely, emphasizing the metabolic importance of reclaiming initiator methionine for cellular reuse. Methionine aminopeptidases (MetAPs) share a conserved structural framework that reflects their ancient evolutionary origins and essential cellular function. The core catalytic domain, originally elucidated by X-ray crystallography from the Escherichia coli enzyme, weighs approximately 30 kDa and houses two metal ions within its active site. This domain displays an internal symmetry known as the “pita bread fold,” believed to be the result of a primordial gene duplication event. This structural motif is consistent across eubacteria and defines the type 1 isoform of MetAPs. In contrast, archaeal MetAPs carry a distinct homologous variant, termed type 2, which includes a ~65-residue insertion forming an additional helical domain. Eukaryotic organisms express both type 1 and type 2 isoforms, each with characteristic N-terminal extensions that differ in composition and presumed function. While the type 1 N-terminal region contains zinc-finger domains suspected to anchor the enzyme to the ribosome, the type 2 N-terminal region in eukaryotes consists of polyacidic and basic sequences whose role remains uncertain. Methionine aminopeptidases (MetAPs) were initially characterized as Co²⁺-dependent enzymes, based on early studies of bacterial forms. However, subsequent research has revealed remarkable metal flexibility, especially in E. coli MetAPs, which can maintain enzymatic activity with Fe²⁺, Zn²⁺, Ni²⁺, and Mn²⁺. This versatility suggests that metal ion utilization may depend on metal availability in the cellular environment, particularly in prokaryotes. In eukaryotic systems, more specific preferences have emerged—Mn²⁺ appears to be the preferred cofactor for type 2 MetAPs, while Zn²⁺ is likely used by type 1 enzymes under physiological conditions. Although MetAPs were previously believed to require a bimetallic active site, functional activity has been demonstrated with a single metal ion. Across all isoforms and species, the coordinating five amino acid side chains that bind the metal ions are highly conserved, underscoring their essential role in catalysis. Early understanding: Co²⁺ initially identified as the active cofactor in bacterial MetAPs. Metal flexibility: E. coli MetAPs function with Fe²⁺, Zn²⁺, Ni²⁺, Mn²⁺, or Co²⁺. Environmental influence: Prokaryotic metal usage may vary depending on cellular metal availability. Type 1 MetAPs: likely use Zn²⁺, Type 2 MetAPs: favor Mn²⁺. Metal stoichiometry: Though typically bimetallic, MetAPs can function with a single metal ion. Structural conservation: Five metal-coordinating amino acids are conserved across species and isoforms. The substrate specificity of all isoforms is generally aligned with previous descriptions and is significantly influenced by the traits of the penultimate residue. In laboratory experiments, the length of the substrate appears to have some effect, though its significance in living organisms remains unclear. Similarly, various metal ions might also affect substrate selection, although this has not been thoroughly validated. Null mutations (alterations that disrupt gene expression) in yeast demonstrate a degree of redundancy, as cells can thrive with either isoform present, but cannot survive if both are missing. The removal of a single gene in prokaryotes is equally lethal. Nonetheless, there is both direct and indirect evidence suggesting that the two isoforms fulfill different cellular functions. MetAP1 is thought to function as the main processing enzyme, physically linked to the ribosome, prepared to hydrolyze methionine from nascent chains during protein synthesis. Conversely, MetAP2 is presumed to be a soluble enzyme that likely carries out secondary processing for substrates that incorrectly bypass the action of MetAP1. However, it is clearly involved in additional functions as well. This is illustrated by a class of irreversible chemical inhibitors that are highly selective for MetAP2, which cause cell-cycle arrest in endothelial cells (without causing cell death), leading to anti-angiogenesis. These potential treatments are currently being refined for application in cancer therapy. It is believed that this inhibition arises from MetAP2's failure to process a specific protein or set of proteins essential for the mitosis of these cells, although the precise identity of these proteins is still unknown. Downstream sequences likely account for making these particular targets vulnerable to MetAP2 instead of MetAP1. Furthermore, MetAP2 also plays a role in inhibiting the phosphorylation of eIF2a, thus promoting translation. This role is entirely distinct from its catalytic activity and is not connected to its cell-cycle functions, as the affected protein continues to act as a phosphorylation inhibitor. Hence, it is one of many proteins recognized for having dual (and often unrelated) roles. Aminopeptidases are essential enzymes involved in critical cellular processes such as protein maturation, peptide activation, and degradation. Among them, methionine aminopeptidases (MetAPs) serve as a key system for N-terminal cotranslational processing, helping to ensure proper protein structure and function across all domains of life. These enzymes show remarkable substrate specificity, and their activity is influenced by the penultimate residue of the target protein as well as the availability of metal cofactors like Zn²⁺, Mn²⁺, and Co²⁺. Structurally, they feature a pita bread fold and maintain conserved active-site residues, pointing to their evolutionary importance. MetAP1: ribosome-associated, processes nascent proteins, MetAP2: soluble, involved in post-translational processing and cell-cycle regulation, Importantly, MetAP2 has been linked to angiogenesis inhibition, making it a promising anticancer drug target. Its ability to affect eIF2α phosphorylation shows it also performs non-catalytic regulatory functions, independent of its enzymatic activity. Protein maturation, Cotranslational processing, Metal ion dependence, Isoform-specific roles (MetAP1 & MetAP2), Therapeutic potential (anti-angiogenesis, cancer therapy), Dual functionality (enzymatic and regulatory). In summary, further exploration of aminopeptidases, especially MetAPs, will enhance our understanding of protein regulation and may lead to targeted treatments for complex diseases. Aminopeptidases are enzymes that cleave amino acids from the N-terminal end of peptides or proteins. Their primary functions include: Protein maturation during or after synthesis, Activation or inactivation of bioactive peptides, Protein degradation, converting peptides into free amino acids, These functions help regulate cellular processes, signaling pathways, and protein turnover. Methionine aminopeptidases (MetAPs) are a specific type of aminopeptidase responsible for removing the initiator methionine from newly synthesized proteins. This step is essential for: Enabling post-translational modifications, Maintaining protein stability and function, Recycling methionine, an amino acid critical for cell survival, MetAPs are conserved across all organisms and are critical for cell viability. Aminopeptidases are produced in various tissues and cells throughout the body. They exist in: Soluble and membrane-bound forms, Cytoplasmic, lysosomal, and extracellular environments, Organs such as the liver, kidneys, small intestine, and immune cells, Their location and form often determine their specific physiological role. Notable examples of aminopeptidases include: Methionine aminopeptidase (MetAP) – initiator methionine removal, Leucine aminopeptidase (LAP) – involved in protein turnover, Aminopeptidase N (CD13) – plays roles in antigen processing and viral entry, Aminopeptidase A (APA) – involved in blood pressure regulation via angiotensin metabolism. Yes, several aminopeptidase inhibitors have been identified and are used in research and therapeutics. These inhibitors: Help study enzyme mechanisms and substrate preferences, Serve as potential anti-cancer or anti-hypertensive drugs, Include compounds like bestatin (broad-spectrum AP inhibitor) and TNP-470 (selective MetAP2 inhibitor). Such inhibitors are being explored for conditions involving abnormal cell growth, such as tumors, or immune dysfunctions.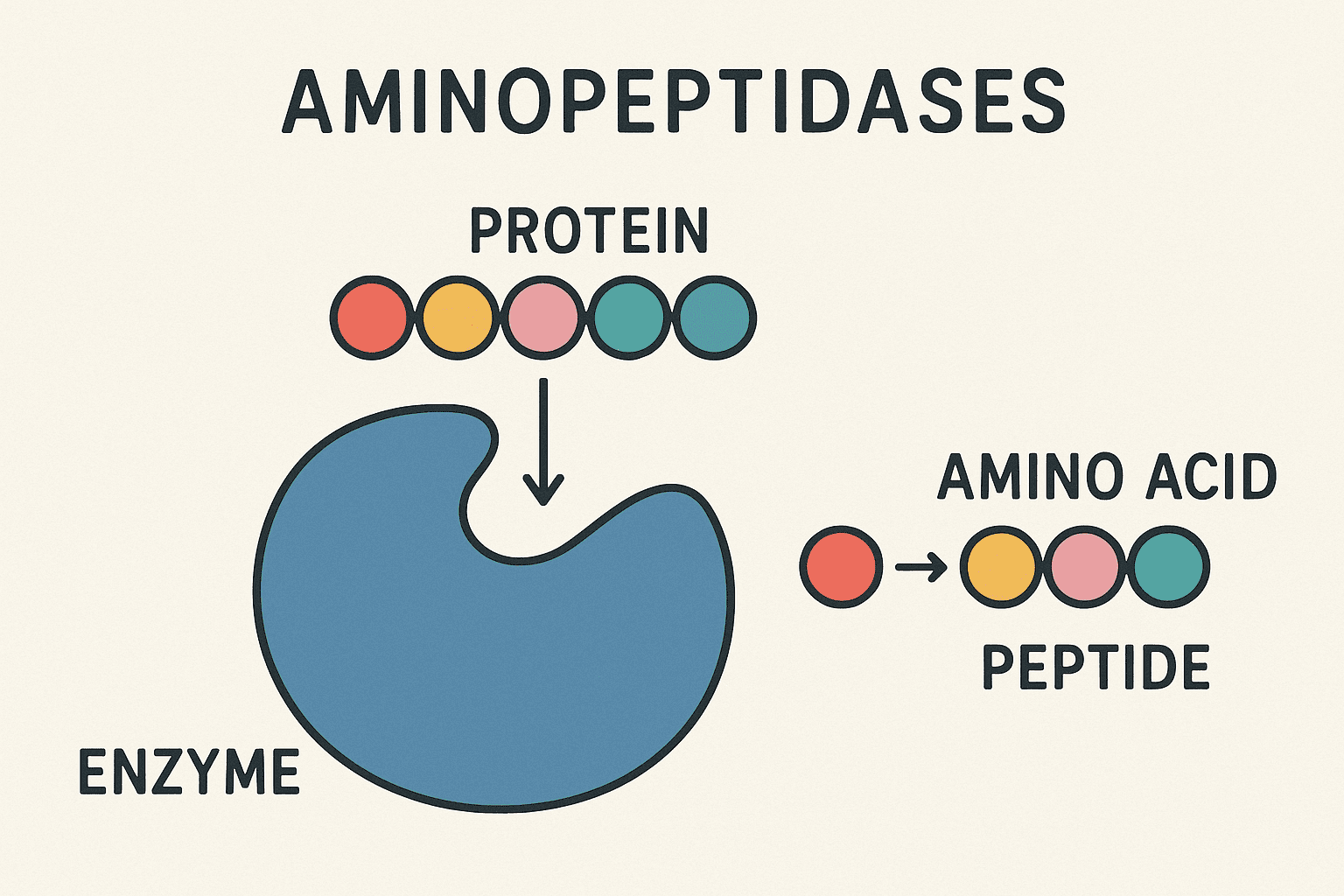
1. Introduction
2. Properties of Aminopeptidases
3. Functions of Aminopeptidases
3.1 Major Functional Categories:
1. Processing and Maturation
2. Activation
3. Degradation
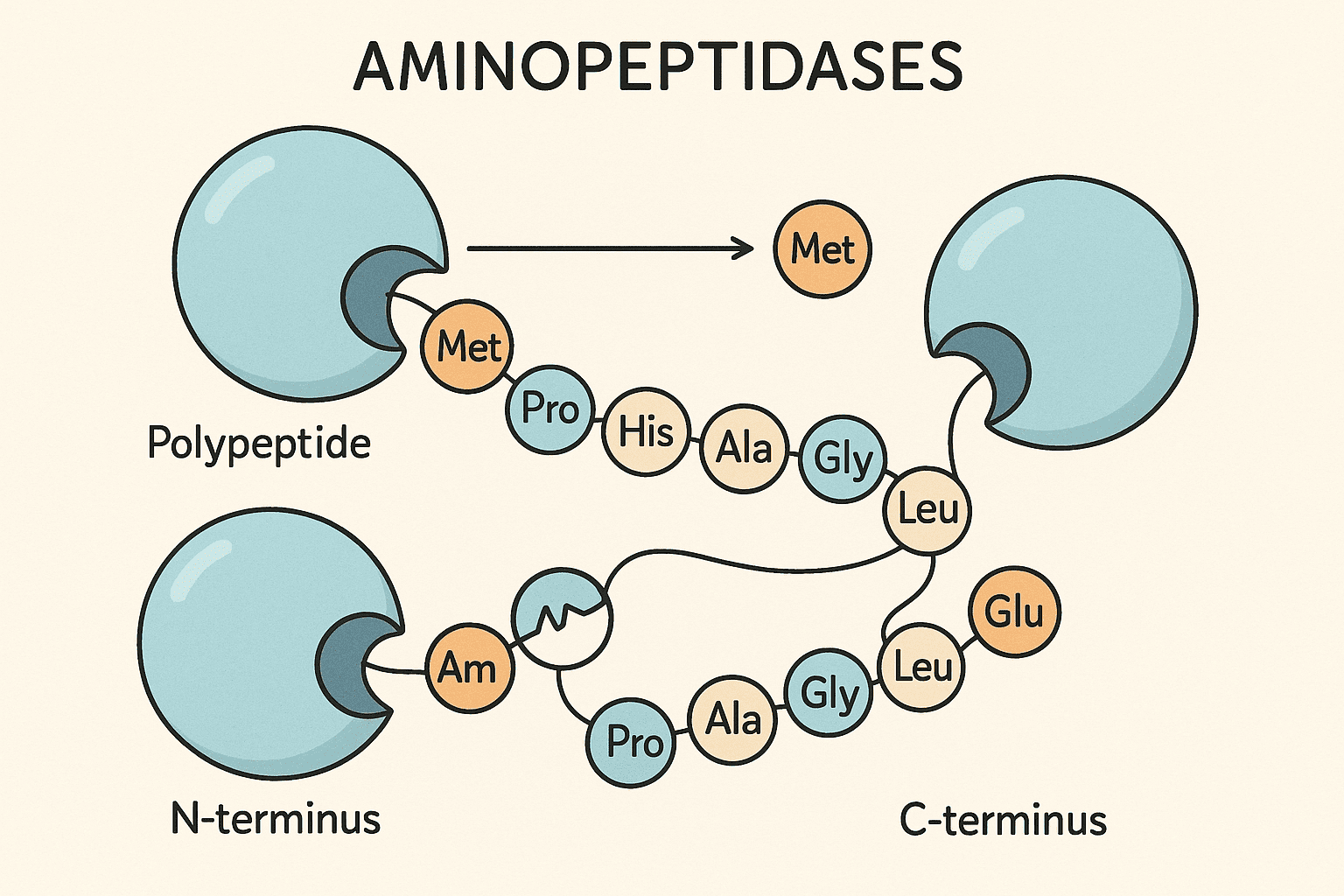
4. N-Terminal Cotranslational Processing
4.1 Why N-terminal Methionine Removal Is Essential:
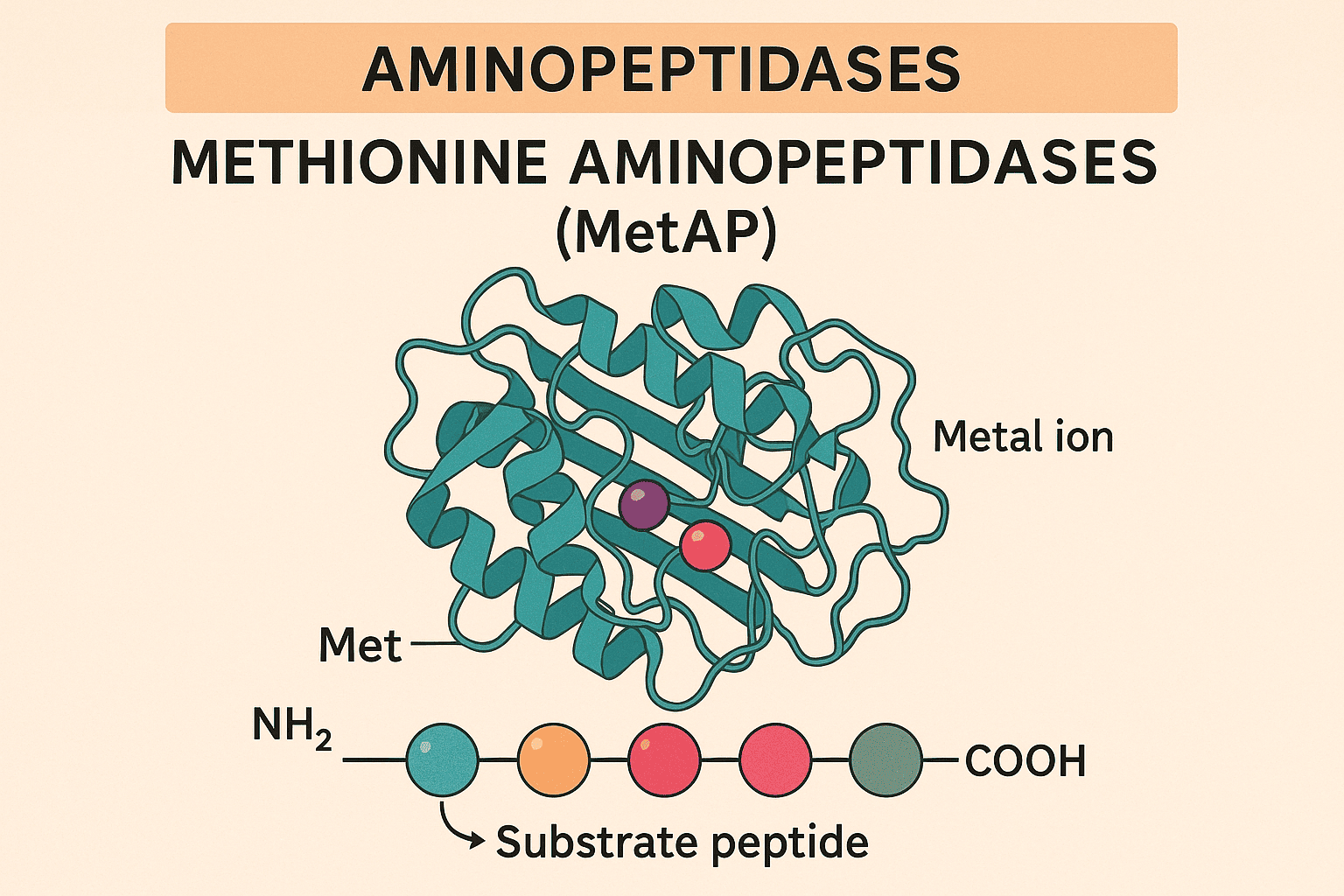
5. MetAP Specificity
5.1 Key Features of MetAP Substrate Specificity:
1. Preferred penultimate residues:
2. Genomic impact in yeast:
3. Trafficked proteins:
4. Biochemical significance:
6. Properties of MetAPs
6.1 Structural Organization
7. Metal Use
7.1 Key Insights on Metal Use in MetAPs:
Eukaryotic specificity:
8. Roles of the MetAP Isoforms
9. Conclusion
9.1 MetAP isoforms exhibit distinct cellular roles:
Key points:
10. FAQs
1. What is the primary function of aminopeptidases?
2. What are methionine aminopeptidases (MetAPs) and why are they important?
3. Where are aminopeptidases produced in the body?
4. What are some examples of aminopeptidase enzymes?
5. Are there inhibitors of aminopeptidases, and what are their uses?
Recent Posts
Sequence Alignment plays a vital role in the subsequent analysis of NGS data, where millions of sequenced DNA fragments (reads) need to be aligned with a chosen reference sequence in a timely manner.
FASTQ files serve as the “raw data files” for any sequencing application, indicating that they are “unaltered.” Consequently, this file format is utilized for performing Quality Checks on sequencing reads. The Quality Check process is typically carried out using the FastQC tool developed by Simon Andrews from Babraham Bioinformatics.
FASTA format is a text-oriented format utilized for depicting either nucleotide sequences or peptide sequences, where nucleotides or amino acids are denoted by a single-letter code.
Mammalian expression systems enable the production of complex, functional recombinant proteins with proper folding and post-translational modifications. These systems are ideal for studying human proteins in a near-native environment, offering advantages in scalability, gene delivery, and purification. HEK293 and CHO cells remain the most widely used hosts, supporting both transient and stable expression strategies for academic and pharmaceutical applications.
Gas Chromatography (GC) stands as one of the most powerful and versatile analytical techniques used to separate and analyze compounds in complex mixtures. At its core, GC enables the identification and quantification of chemical substances based on their molecular composition and retention behaviors during migration through a chromatographic column.